Of a liquid heated to the point when it starts to turn into a gas. Updated September 05 2019.
Boiling Point Melting Point Heat Temperature Chemistry Png Clipart Angle Boil Boiling Chemical Substance Diagram Free
Boiling is used primarily to cook meats and vegetables.

Definition of boiling in chemistry. This occurs when the liquids vapor pressure. The boiling point of a liquid is the temperature at which the vapour pressure of the liquid becomes equal to the atmospheric pressure of the liquids environment. Glossary of chemistry terms.
The temperature at which the vapor pressure of a liquid is equal to the pressure of the atmosphere on the liquid equal to 212F 100C for water at sea level. Meaning and definition of boiling tube. The temperature at which vapour 346C 783C100C1 pressure of a liquid becomes equal to the atmospheric pressure or any other external pressure on the liquid is called boiling point of that liquid.
Thus you see it is the rapid vaporization of a liquid when it reaches its set boiling point. The boiling point becomes lower as the external pressure is reduced. What is the definition of the boiling point in chemistry.
Moreover it produces bubbles. Boiling is defined as a phase transition from the liquid state to the gas state usually occurring when a liquid is heated to its boiling point. Water-soluble substances such as sugar and salt raise the boiling point.
At the boiling point the vapor pressure of the liquid is the same as the external pressure acting upon its surface. Therefore the boiling point of a liquid depends on atmospheric pressure. Closed last year.
Boiling is when a liquid becomes a vapor by touching a hot surface. The point when bubbles appear The point when a liquid turns into a gas The point when the vapor pressure of a liquid equals the. The point at which matters reach a crisis.
That temperature is very commonly referred to as the liquids normal boiling point. At this temperature the liquid is. Illustrated Glossary of Organic Chemistry.
The temperature at which the vapor pressureof a liquid equals the ambient pressure. It occurs when the heat is at the boiling point. What does boiling point mean in social studies.
But how does the vapour pressure of the liquid form above the liquid when the surrounding the portion just above the liquid is having the atmospheric pressure. Condensation pointis the temperature at which a vapor condenses into a liquid without a change in the temperature of the substance. 2 The temperature at which the vapor pressure of a liquid is equal to or slightly greater than the atmospheric pressure of the environment.
A liquid at high pressure has a higher boiling. Boiling is when any liquid turns into gas when you heat it continuously. The definition of boiling point says boiling point is the temperature at which the vapour pressure of liquid becomes equal to vapour pressure of atmosphere.
Learn about the uses and methods of boiling. Boiling the cooking of food by immersion in water that has been heated to near its boiling point 212 F 100 C at sea level. A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure.
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor. Also the condensation point. A vapor is a gas which is formed by the boiling or evaporation of a liquid.
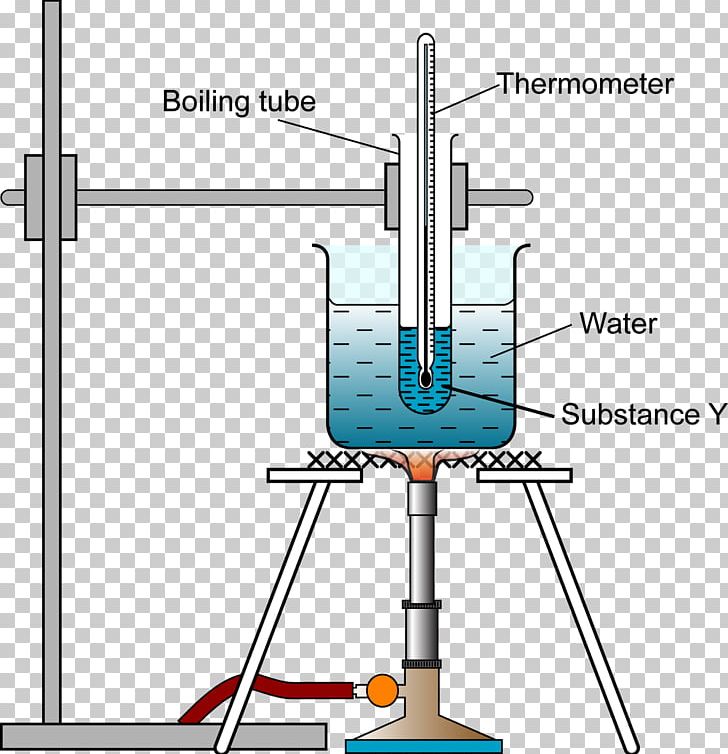
A thin glass tube closed at one end and used for chemical tests etc. The boiling point of a liquid varies depending upon the surrounding environmental pressure. Boiling or the boiling point is the temperature when a liquid will have vaporization occurring through the entire liquid not just on the surface.
The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. During boiling vapor must escape from the solid.
Under this condition addition of heat results in the transformation of the liquid into its vapor without raising the temperature. For water at sea level its boiling point is 100C. The point beyond which one becomes angry outraged or agitated.
The composition and thickness of the glass is such that it cannot sustain very high temperatures and is intended for heating liquids to boiling point. The atmospheric pressure the liquid has reached its boiling point namely the temperature at which the liquid changes its state from a liquid to a gas throughout its bulk. Boiling point temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid.
The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. 1 The temperature at which the vapor pressure of a liquid is equal to the applied pressure. Definition and Explanation of Boiling Point.
States Of Matter The Kinetic Theory Of Matter Boiling Point Evaporation And Melting Point Chemistry Hive

Boiling Definition In Chemistry Study Com
Difference Between Vapor Pressure And Boiling Point Definition Conditions Characteristics

Boiling Point Definition Examples Temperature Facts Britannica

Boiling Point Elevation Chemistry For Non Majors

Vapour Pressure And Boiling Point Urdu Hindi Chemistry Class 9 Class 11 Youtube Youtube
.png?revision=1&size=bestfit&width=1073&height=358)
1 4b Controlled Boiling Chemistry Libretexts

Boiling Point External Pressure Liquid And Gases Chapter No 4 Chemistry Part 1 Youtube
