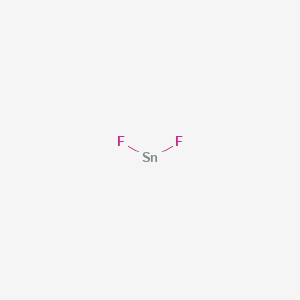Complete these in lab and on your own time for practice. You should complete this by Sunday.

How To Write The Formula For Tin Ii Fluoride Youtube
E-mail to a friend.
Tin fluoride formula. TinII fluoride commonly referred to commercially as stannous fluoride from Latin stannum tin is a chemical compound with the formula SnF 2. Fe2CrO43 iron III chromate 46. Mercury I chloride Hg2Cl2 33.
Name the following compounds include Roman Numerals when necessary. And for each compound they all have a molecular formula but some can be similar and those are called isomers which are common in organic chemistry. Copy this to my account.
SnO 2 tin IV oxide Sb 2S 5 antimony V sulfide Au 3P gold I phosphide AsH 3 arsenic III hydride. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. This is different than most medicines used for weak bones osteoporosis which fight osteoporosis by.
8 water molecules octahydrate. In a molecular formula it states the total number of atoms of each element in a molecule. Iron II fluoride FeF2 37.
Use these pages as a study guide. Copper I nitride Cu3N 34. SnSO42 tin IV sulfate 55.
ZnBr2 zinc bromide 45. In addition you should know names and symbols for all elements in Groups I and II as well as the halogens Group VII noble gases Group VIII and some other metals used frequently. Ionic Compound Naming and Formula Writing List 1.
Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. The suffix -ic refers to the form with a higher ionic charge while the suffix -ous refers to the form with the lower ionic charge. Potassium chloride KCl 36.
Zinc iron II iron III gallium silver lead IV chloride ZnCl 2. With the formula AlF 3 xH 2 O these compounds include monohydrate x 1 two polymorphs of the trihydrate. Sodium chloride NaCl and magnesium oxide MgO.
Ionic Compound Formula K sp. The chemical formula of ionic compounds can be quickly calculated using the chemical formula calculator. The first box is the intersection between the zinc cation and the chloride anion so you should write ZnCl 2 as shown.
PbO2 lead IV oxide 56. Na Mg 2 Non. The names are found by finding the intersection between the cations and anions.
H 2 PO 4-Sulfate. TinIV stannateIV Ligands are named before the central metal atom. When the chemical formula for a hydrated ionic compound is written the formula for the ionic compound is separated from the waters of hydration by a centered dot.
Common Covalent Binary Inorganic Compounds of atoms Prefix element closest to fluorine goes on rightCommon Examples 1 Mono H 2 Hydrogen N 2 Nitrogen 2 Di O 2 Oxygen NH 3 Ammonia 3 Tri O 3 Ozone NO Nitrogen monoxide Nitric Oxide 4 Tetra H 2O Water Dihydrogen Monoxide NO 2 Nitrogen dioxide 5 Penta F 2 Fluorine N 2O Dinitrogen monoxide Nitrous oxide 6 Hexa HF Hydrogen fluoride N. Copy this to my account. Cu2S copper I sulfide 43.
For example the molecular formula of glucose is C_6H_12O_6 and we do not simplify it into CH_2O. Cations Anions zinc iron II iron III gallium silver lead IV chloride. Ionic Compounds Naming and Formula Writing.
Solubility Product Constants near 25 C. For example many fluoride rinses list stannous fluoride as an ingredient. Hg2Cl2 mercury I chloride 57.
Stannous fluoride was introduced as an alternative to. It also promotes new bone formation. If the formula is given write down the name and if the name is given write down the formula.
Chemical Formula Nomenclature Practice. Tin IV sulfide SnS2 35. The molecular formula for glucose is C₆H₁₂O₆ which tells us the exact number of constituent atoms carbon hydrogen and oxygen written as C H and O respectively in one glucose molecule.
Cadmium fluoride CdF 2 silver sulfide Ag 2S potassium phosphide K 3P zinc carbide Zn 2C. Tin II sulfide SnS 31. E-mail to a friend.
Tin II oxide SnO 42. Fluoride protects teeth from the bacteria in plaque. Stannous refers to tinII so the chemical formula for stannous fluoride is SnF 2.
Mercury I iodide Hg2I2 40. Structural formula of glucose will indicate how each carbon hydrogen and. BaCIO32 barium chlorate 53.
Sodium thiosulfate ____Na2S2O3_____ 3. Other commonly used nonstandard names include ferric ironIII ferrous ironII and stannic tinIV. The transfer of electrons between metals and non-metals produces charged particles called ions.
Aside from anhydrous AlF 3 several hydrates are known. Identify the central metal ion Identify the oxidation state on the central metal ion shown in Roman numerals parantheses Identify the ligands Identify the number of ligands Calculate the total charge on the ligands Calculate the charge on the complex ion oxidation. Aluminium fluoride refers to inorganic compounds with the formula AlF 3 xH 2 O.
AsO 3 3-Hydrogen phosphate. Metals lose electrons to produce positve ions called cations. They are all colorless solids.
The names are found by finding the intersection between the cations and anions. Several occur as minerals. Tin IV oxide SnO2 39.
The empirical formula of glucose is CH₂O which represents the whole number ratio of the constituent atoms viz C H and O. Use the stock form for the transition metals. Sulfur dioxide ____SO2_____ 2.
Polyatomic Ionic Compounds 1. Mercury II iodide HgI2 32. Aluminum hydroxide AlOH 3 1810 5 Aluminum phosphate AlPO 4 6310 19 Barium carbonate BaCO 3 5110 9 Barium chromate BaCrO 4 1210 10 Barium fluoride BaF 2 1010 6 Barium hydroxide BaOH 2 510 3 Barium sulfate BaSO 4 1110 10 Barium sulfite BaSO 3 810 7 Barium thiosulfate BaS 2 O 3.
NaCIO4 sodium perchlorate 47. Stannous Fluoride or TnII Fluoride is a compound commonly used in toothpastes for the prevention of gingivitis dental infections cavities and to relieve dental hypersensitivityAlthough similar in function and activity to Sodium Fluoride NaF the conventionally added ingredient in toothpastes stannous fluoride has been shown to be more effective at stopping and reversing dental lesions. Strontium bromide SrBr2 44.
Chemical Formula Writing Worksheet Two Write chemical formulas for the compounds in each box. Lithium phosphide Li3P 41. Na 2SO 4 sodium sulfate AℓPO 4 aluminum phosphate Aℓ C ℓO 4 3.
An ionic compound is composed of a metal and a non-metal. HPO 4 2-Dihydrogen phosphate. Give the formula for the following.
Al2S3 aluminum sulfide 54. S 2 O 3 2-Sulfite. Ionic Compound Formula Writing Worksheet Write chemical formulas for the compounds in each box.
Anhydrous AlF 3 is used in the production of aluminium metal. The first box is the intersection between the zinc cation and the chloride anion so you should write ZnCl 2 as shown. 6 Writing the Line Formula of a Complex.
As a general rule you should know the names and symbols for elements 1-36. It is a colourless solid used as an ingredient in toothpastes Oral health benefits. MnClO44 manganese IV perchlorate 44.
MgF2 magnesium fluoride 52. Greek prefixes are attached to the word hydrate to indicate the number of water molecules per formula unit for the compound eg BaOH 2 8H 2 O.
Stannous Fluoride Snf2 Pubchem

Stannous Fluoride Facts Formula Properties Uses

Tin Iv Fluoride 99 Metals Basis Thermo Scientific 10g Tin Iv Fluoride 99 Metals Basis Thermo Scientific Fisher Scientific
Tin Tetrafluoride F4sn Chemspider
Sn F 2 Formula Tin Ii Fluoride Plumbic