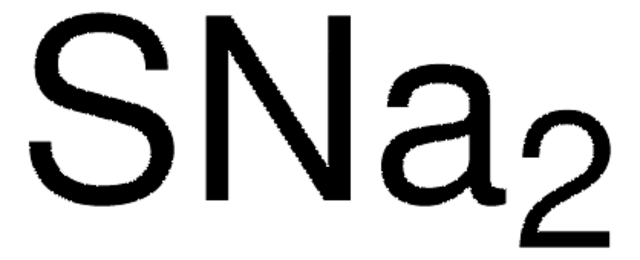3D left and center and 2D. 36 Full PDFs related to this paper.
UOP202-14 Disulfide Sulfur in Light Petroleum Distillates and LPG.

Molecular mass of sodium sulfide. METHOD 6013 Issue 1 dated 15 August 1994 - Page 2 of 4 SAMPLING. The model should show 27 protons and 32 neutrons B. The model should show 59 protons and 27 neutrons.
Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. Determine the molar mass of sodium oxide Na 2 O.
Which uses a molecular sieve sampler but has poor stability. Molecular mass of H 2 O 2 x atomic mass of H 1 x atomic mass of O 2 x 100797 1 x 159994 amu 1802 amu. An organic compound is known to be composed of 4660 carbon 6800 hydrogen and 4660 oxygen.
Yes because the chemical factor 15921271 is just a bit greater than unity indicating that the mass of the product will slightly exceed that of the copper. This effect could also be demonstrated by preincubating cells for 3-20 min within 5-10 mM sodium hydrosulfide. The molecularformula mass is numerically equal to the mass of one mole of the substance.
Production of hydrogen sulfide can be detected when ferrous sulfide a black precipitate is produced as a result of ferrous ammonium sulfate reacting with H2S gas. The atomic mass of chlorine is 3545 g per mole while the atomic mass of sodium is 2299 g per. Sodium sulfide anhydrous is a yellow to brick red crystalline mass or fused solid with an odor of rotten eggs.
NIOSH Manual of Analytical Methods NMAM Fourth Edition 81594. A student wants to create a model of a cobalt atom. Sodium bicarbonate baking soda NaHCO3 can be purified by dissolving it in hot water 60 C filtering toexample.
As an example if you need to do figure out the molar mass of NaCl youll have to find the atomic mass of both sodium and chlorine. Determine the molar mass of ozone. For network solids the term formula unit is used in stoichiometric calculations.
The molecular mass can be calculated from the chemical formula and is expressed in conventional atomic mass units equal to 112 of the mass of a neutral carbon-12 12 C isotope atom. Is this answer reasonable. Doing this should give you the molar mass of a compound which is comprised of various kinds of atoms.
With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. Molecular mass molecular weight is the mass of one molecule of a substance and is expressed in the unified atomic mass units u. Patch clamp studies of neuroblastoma cells have shown that in the presence of sodium hydrogen sulfide the in vitro precursor of hydrogen sulfide addition of the sulfonated amino acids taurine or cysteic acid resulted in reversible abolition of the inward of the sodium currents.
1 mole molar mass could be atomic mass from periodic table or molecular mass 1 mole 224 L of a gas at STP You do not need to worry about this yet Each definition can be written as a set of two conversion factors. The relations among amount of substance in. Substance MW or FW molar mass Fe 55.
Type 13X offers enhanced adsorption performance over the type A zeolite and it can remove impurities too large to fit into. 00 amu 3 The molecular mass of H2SO4 98. 1 mole molar massg can be written as ____1 mole OR _molar mass g.
H 3 x 1. Sodium and aluminium are taken to compare. UOP212-05 Hydrogen Sulfide Mercaptan Sulfur and Carbonyl Sulfide in Hydrocarbon Gases by Potentiometric Titration.
We would like to show you a description here but the site wont allow us. Determine the molar mass of calcium sulfide CaS. 1 Cobalt has a mass number of 59 and an atomic number of 27.
The mass of copper sulfide formed will be determined by the mass of copper available. Sodium iodide chemical formula NaI is an ionic compound formed from the chemical reaction of sodium metal and iodineUnder standard conditions it is a white water-soluble solid comprising a 11 mix of sodium cations Na and iodide anions I in a crystal latticeIt is used mainly as a nutritional supplement and in organic chemistryIt is produced industrially as the salt formed when. Ammonium hydroxide solution 25.
The model should show 27 protons and 27 neutrons. A Laboratory Manual Third Edition 1982. If exposed to moist air it is liable to spontaneous heating and may cause ignition of nearby combustible material.
The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. Which statement about the model is correct1 point A. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H.
Beef extract 30 g Peptone 300 g Ferrous ammonium sulphat 02 g Sodium thiosulphate 0025 g Agar 30g Final pH at 25C 7302 Distilled water 1000ml. UOP209-00 Alkalinity Sulfide and Mercaptide Analyses of Used Refinery Caustic Solutions. So melting point of aluminium is greater than sodium.
Sodium loses its outer electron to give it a stable electron configuration. What is its empirical formula. CO3- because the background.
Molecular mass - when molecular mass increase. Molar mass has units of grams per mole gmol. 4 To obtain one mole of copper atoms 602 x 10 23 atoms weigh out 6355 g copper.
Sodium is s block metal and aluminium is a p block metal. Its monatomic form H is the most abundant chemical substance in the Universe constituting roughly 75 of all baryonic mass. Due to release of three electrons and less radius metallic lattice of aluminium is much stronger than sodium.
Hydrogen peroxide 30 3. How would you calculate the empirical formula of acetic acid CH3COOH. It also has the highest theoretical capacity of the common adsorbents and excellent mass transfer rates.
UOP163-10 Hydrogen Sulfide and Mercaptan Sulfur in Liquid Hydrocarbons by Potentiometric Titration. 15 g Cu 1592 g Cu 2 S 1271 g Cu 188 g Cu 2 S Check. With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table.
5 The molar mass M of a substance is the mass of one mole of entities atoms molecules or formula units of the substance. HYDROGEN SULFIDE 6013 H 2S MW. Why d block elements.
Its monatomic form H is the most abundant chemical substance in the Universe constituting roughly 75 of all baryonic mass. But both are located in 3rd period of the periodic table. For example the molecular weight of water would be obtained by the following process.
The relation between molecular formula mass and molar mass. A short summary of this paper. Full PDF Package Download Full PDF Package.
Copper will react with sulfur to form a copper sulfide if 1500g copper reacts with sulfur to form 1880g of copper sulfide what is the empirical formula of the copper sulfide. Science CHECK MY ANSWERS. The 13X molecular sieve is the sodium form of zeolite X and has a much larger pore opening than the type A crystals with an effective pore diameter of 10 angstroms.
A Laboratory Manual Third Edition. A Laboratory Manual Third Edition.

Molar Mass Molecular Weight Of Na2so4 Sodium Sulfate Youtube

Molar Mass Molecular Weight Of Na2s Sodium Sulfide Youtube

Molar Mass Molecular Weight Of Na2s Sodium Sulfide Youtube
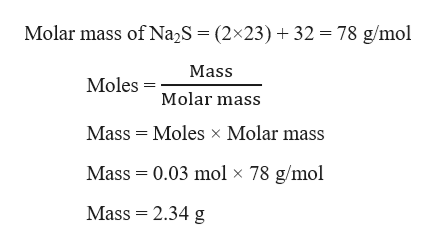
Answered How Many Grams Of Sodium Sulfide Are Bartleby

Sodium Sulfide 1313 82 2 Tokyo Chemical Industry Co Ltd Jp

Calculate Molecular Mass Of Na2s Molar Mass Of Na2s Sodium Sulfide Molar Mass Youtube