The clinical significance of acid-base balance is one which is hard to deny. 2 Al 6 HCl 3H 2 2 AlCl 3 Single Replacement 6.

Is K3po4 Acidic Basic Or Neutral Dissolved In Water Youtube
Aldosterone is the main mineralocorticoid steroid hormone produced by the zona glomerulosa of the adrenal cortex in the adrenal gland.

Potassium phosphate acid or base. Maaden mine Phosphate Rock and process it into diversified ammonium Phosphate Fertilizers Products. May result from excessive potassium loss by the renal or gastrointestinal route from decreased intake or from transcellular shifts. TABLE PageIndex1Important Conjugate Acid-Base Pairs.
It plays a central role in the homeostatic regulation of blood pressure plasma sodium Na and potassium K levelsIt does so primarily by acting on the. With 20 times more bicarbonate than carbonic acid this capture system is most efficient at buffering changes that would make the blood more acidic. Acid excretion in the urine can be estimated by a formula described by Remer sulfate chloride 18x phosphate organic acids minus sodium potassium 2x calcium 2x magnesium mEq.
Calcium Hydroxide and Phosphoric acid react to form Calcium Phosphate and water. It can be used in fertilizer mixtures to reduce escape of ammonia by keeping pH low. MRNA lipids 4-hydroxybutylazanediylbishexane-61-diylbis2-hexyldecanoate 2 polyethylene glycol-2000-NN-ditetradecylacetamide 12-Distearoyl-sn-glycero-3-phosphocholine and cholesterol potassium chloride monobasic potassium phosphate sodium chloride dibasic sodium phosphate dihydrate and sucrose.
1985The chemical shift of 31P in these two molecules differs by approximately 24 ppm but rapid exchange between the. Gold. Wilson and Green 1985.
An exception to this is in acid-base disorders which can be caused by or lead to changes in chloride levels independent of sodium. Bicarbonate ions and carbonic acid are present in the blood in a 201 ratio if the blood pH is within the normal range. Acid-base balance in the human body is one of the most paramount physiological processes.
As with the phosphate buffer a weak acid or weak base captures the free ions and a significant change in pH is prevented. Dietary Potassium Potassium phosphate. DBP Market Monthly Report 202110 Oct 29.
We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. The market is temporarily stable and wait-and-see for 02Nov 2021. Measurements of Intracellular pH Inorganic phosphate has a chemical shift that is pH-dependent.
The use of conjugate acid-base pairs allows us to make a very simple statement about relative strengths of acids and bases. With 20 times more bicarbonate than carbonic acid this capture system is most efficient at buffering changes that would make the blood more acidic. Bicarbonate ions and carbonic acid are present in the blood in a 201 ratio if the blood pH is within the normal range.
Phosphates are a naturally occurring form of the element Phosphorus one of the three primary nutrients with Nitrogen and Potassium are the other two required for photosynthesis and crop growth. Some of the most common admissions to hospitals are due to diseases that can dangerously affect the acid-base balance. The component ions in a salt compound can be either inorganic such as.
Monopotassium phosphate MKP also potassium dihydrogenphosphate KDP or monobasic potassium phosphate KH2PO4 is a soluble salt of potassium and the dihydrogen phosphate ion. See Hyperkalemia. This is why it is important for clinicians to understand basic principles which govern this portion of.
The stronger an acid the weaker its conjugate base and conversely the stronger a base the weaker its conjugate acid. PfizerBioNTech The full list of ingredients for the Pfizer vaccine is. Consists of a weak acid and its base or salt.
The urine may have a variable pH from acid to alkaline depending on the need for balancing the internal environment. As with the phosphate buffer a weak acid or weak base captures the free ions and a significant change in pH is prevented. Cl-is involved in water balance maintaining osmotic pressure and acid-base balance.
Abnormally low potassium concentration in the blood. It is a source of phosphorus and potassium as well as a buffering agent. The reason for this is that at neutral pH Pi exists principally as HPO4 and H2PO4 with an acid-base dissociation constant pK acid of 677 in brain tissues Petroff et al.
Evaluation of mixed acid-base abnormalities requires an understanding of the anion gap the relationship between the change in serum sodium and chloride concentration and the limits of compensation for the primary acid-base imbalances Saxton and Seldin 1986. Potassium metal and Chlorine gas combine to form 2 K Cl 2 2KCl Synthesis 5. Copper and Sulfuric acid react to.
Aluminum and Hydrochloric acid react to form Aluminum Chloride and Hydrogen gas. HHB acid hemoglobin KHBpotassium hemoglobin KH 2 PO 4 potassium acid phosphate K 2 HPO 4 potassium alkaline phosphate H Protein acid porteinate NA protein sodium proteinate NaH 2 PO 4 sodium acid phosphate NaHPO 4 sodium alkaline phosphate Closed system as no gas is available to. Clinical findings and history are also necessary to define the factors that may contribute to the development.
Daily Review of Ethyl Acetate. Potassium Phosphate Dibasic is the dipotassium form of phosphoric acid that can be used as an electrolyte replenisher and with radio-protective activityUpon oral administration potassium phosphate is able to block the uptake of the radioactive isotope phosphorus P 32 P-32. A common example is table salt a molecule of which has a positively charged sodium ion and a negatively charged chloride ion.
3 CaOH 2 2 H 3 PO 4 Ca 3 PO 4 2 6H 2 O Double Replacement 7. Disorders of chloride balance. Table PageIndex1 gives a list of some of the more important.
Shandong Yuanli Technologys DBP Device Dynamics 01 Nov 2021 Nov 1. In chemistry a salt is a chemical compound consisting of an ionic assembly of a positively charged cation and a negatively charged anion which results in a compound with no net electric charge. It is essential for sodium conservation in the kidney salivary glands sweat glands and colon.
Hyperchloremia Cl- 105 mgdL Normal anion gap metabolic acidosis. Manifested clinically by neuromuscular disorders ranging from weakness to paralysis by electrocardiographic abnormalities and by renal and gastrointestinal.

Is It Possible For Monobasic And Dibasic Potassium Phosphate To Complete Break Down Into Phosphates Chemistry Stack Exchange

How Can Prepare Potassium Phosphate Buffer Ph 8

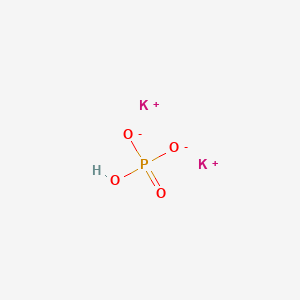
Tripotassium Phosphate Wikipedia
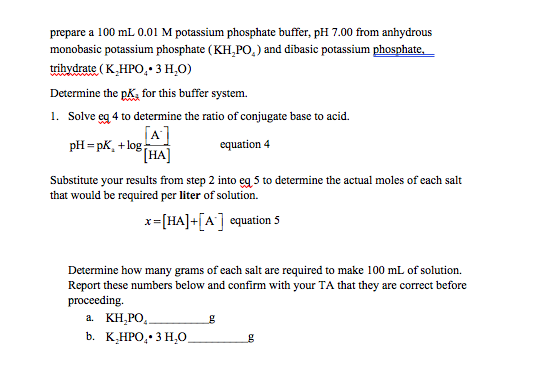
Solved Prepare A 100 Ml 0 01 M Potassium Phosphate Buffer Chegg Com

Potassium Phosphate An Overview Sciencedirect Topics

Is It Possible For Monobasic And Dibasic Potassium Phosphate To Complete Break Down Into Phosphates Chemistry Stack Exchange
Dipotassium Hydrogen Phosphate K2hpo4 Pubchem
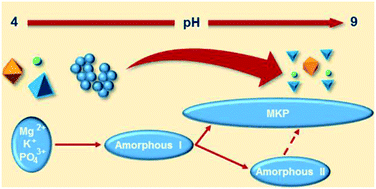
Polyamorphism And Frustrated Crystallization In The Acid Base Reaction Of Magnesium Potassium Phosphate Cements Crystengcomm Rsc Publishing
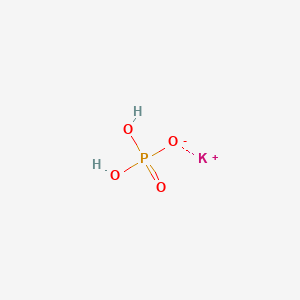
Potassium Dihydrogen Phosphate Kh2po4 Pubchem