The balanced chemical equation for this reaction is 2 PbNO 3 2 s produces 2 PbO s 4NO 2 O 2 g. CID 1118 Sulfuric acid CID 5352425 Lead Dates.
In lead II iodide the charges balance in a 1.
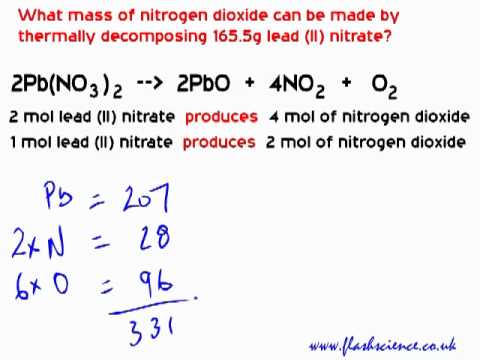
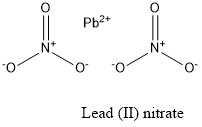
Lead ii nitrate formula. Zinc phosphate Zn3PO42 4. Example- PbNO 3 4 write the name lead nitrate. Bisulfate HSO 4.
An ionic compound is composed of a metal and a non-metal. When leadII nitrate is heated it decomposes into lead II oxide nitrogen dioxide and oxygen. Following are the easy steps to balance the equation.
E-mail to a friend. Na2CrO4 sodium chromate 19. 1 ratio so potassium iodide is simply KI.
The material is soluble in water. ONE-SCHOOLNET Periodic Table. Nitrate NO3-nitrite NO2-oxalate C2O4 2-perchlorate ClO4-periodate IO4-permanganate MnO4-peroxide O2 2-phosphate PO4 3-phosphite PO3 3-silicate SiO4 4-sulfate SO4 2-sulfite SO3 2-thiocyanate SCN-thiosulfate S2O3 2-Other Ions copper I cuprous Cu copper II cupric Cu2 iron II ferrous Fe2 iron III ferric Fe3 lead II plumbous Pb2.
It was formerly called plumbous iodide. Cadmium phosphate Cd3PO42 9. The compound currently has a few specialized applications such as the manufacture of solar cells and X-ray and gamma-ray detectors.
Sodium chloride NaCl and magnesium oxide MgO. S 2 O 3 2. Na Mg 2 Non.
E-mail to a friend. Cu2CO3 Section B Write the formula of the ionic compounds containing polyatomic ions 1. Its great importance comes from the fact that the salt.
SrNO32 strontium nitrate 27. The chemical formula of ionic compounds can be quickly calculated using the chemical formula calculator. LeadII iodide or lead iodide is a salt with the formula PbI 2At room temperature it is a bright yellow odorless crystalline solid that becomes orange and red when heated.
Details of the supplier of the safety data sheet Emergency Telephone Number For information US call. 10 MnNO 33 is manganese III nitrate 11 FePO 4 is iron III phosphate 12 CoCO 3 is cobalt II carbonate Name to formula problems. Ionic Compounds Naming and Formula Writing.
Learn about the health effects of lead who is at risk how to test for lead in paint or other areas of your home how to find or become a lead-safe certified firm and more about the Lead Renovation Repair and Painting RRP rule. AN is an inorganic salt with colorless and odorless crystals that are soluble in most organic solvents. The other product of the reaction is aqueous sodium nitrate NaNO3 which will exist as ions in solution.
Known since the Middle Ages by the name plumbum dulce the production of leadII nitrate from either metallic lead or lead oxide in nitric acid was small-scale for direct use in making other lead. Since lead has more than one oxidation state we must figure out which lead we have. CID 944 Nitric acid CID 5352425 Lead Dates.
LeadII nitrate is an inorganic compound with the chemical formula PbNO 3 2. SnOH2 tin II hydroxide 28. It commonly occurs as a colourless crystal or white powder and unlike most other leadII salts is soluble in water.
This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. PbNO3 2 2KI PbI 2 2KNO 3. Uses advised against Food drug pesticide or biocidal product use.
Complete these in lab and on your own time for practice. Lead II nitrate is also called lead nitrate or plumbous nitrate. CHEMISTRY 1A NOMENCLATURE WORKSHEET Chemical Formula Nomenclature Practice.
Metals lose electrons to produce positve ions called cations. Write the Unbalanced Chemical Equation. May be mildly.
H 2 PO 4. When these two solutions are mixed the leadII cations Pb2 and the iodide anions I will bind to each other and form leadII iodide an insoluble ionic compound. Zinc iron II iron III gallium silver lead IV chloride ZnCl 2.
Reaction between lead nitrate and potassium iodide. NiS nickel II sulfide Write the chemical formula for each of the following compounds. PbNO 3 4 is named leadIV nitrate Highlight to reveal names.
So our roman numeral will be IV. Insoluble in water and sinks in water. C 2 O 4 2.
13 potassium fluoride is KF 14 ammonium sulfate is NH 42SO 4 15 magnesium iodide is MgI 2 16 copper II sulfite is CuSO 3 17 aluminum phosphate is AlPO 4 18 lead II nitrite is PbNO 22. The transfer of electrons between metals and non-metals produces charged particles called ions. Copper I sulfite Cu2SO3 8.
Steps to balance chemical equation. Chemical formula plays an important role in understanding different concepts of chemistry. Sodium carbonate Na2CO3 6.
Lead sulfate appears as a white crystalline solid. It was first described in 1659 and currently is the most commercially important ammonium compound in production volume and usage terms. Ionic Compound Formula Writing Worksheet Write chemical formulas for the compounds in each box.
The potassium ion is K and the iodide ion is I-. The salt is hardly found in nature for its high water solubility. Its preparation is an entertaining and popular.
The first box is the intersection between the zinc cation and the chloride anion so you should write ZnCl 2 as shown. Sulfuric acid lead2 salt 11 More. PbCl2 lead II chloride 7.
It is noncombustible but it will accelerate the burning of combustible materials. If large quantities of the material are involved in. Ionic Compounds Naming and Formula Writing.
To balance the charges we require two nitrate ions per lead II ion and so lead II nitrate is PbNO_3_2. 1 copper II chloride CuCl 2 2. Na2S sodium sulfide 17.
The names are found by finding the intersection between the cations and anions. Iron II chloride 18. Strontium acetate SrC2H3O22 3.
Chemistry is all about learning chemical elements and compounds and how these things work together to form several chemical equations that are hard to understand. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. NH42SO4 ammonium sulfate 20.
KNO3 potassium nitrate 8. Nitrate ZnNO 32 FeNO 32 FeNO 33 GaNO 33 AgNO 3 PbNO 34 oxide ZnO FeO Fe 2O3 Ga 2O3 Ag 2O PbO 2 nitride Zn 3N2 Fe 3N2 FeN GaN Ag 3N Pb 3N4 sulfate ZnSO 4 FeSO 4 Fe 2SO 43 Ga 2SO 43 Ag 2SO 4 PbSO 42 Write the formulas for the following compounds. Lead nitrate is a white crystalline solid.
Contact may irritate skin eyes and mucous membranes. Lead II chlorate PbClO32 2. Let us consider an.
Copy this to my account. MgOH2 magnesium hydroxide 9. The entire group 1 metal can react with oxygen to form metal oxide.
Copy this to my account. Product Name LeadII nitrate Cat No. Lead IV dichromate PbCr2O72 7.
AC193320000 AC193320100 AC193320500 CAS-No 10099-74-8 Synonyms Nitric acid lead2 salt. Ammonium cyanide NH4CN 5. LiCIO3 lithium chlorate 10.
Lead dinitrate Recommended Use Laboratory chemicals. Reaction with Oxygen. Anions zinc iron II iron III gallium silver lead IV chloride ZnCl 2 acetate nitrate oxide.
Lead nitrate PbNO32 More. Reaction of Group 1 Elements. The two charges balance in a 1.
Since each nitrate 4 of them has a 1- charge the Pb must be 4. The reaction between lead nitrate and potassium iodide is an example of a precipitation reaction. 2 ratio so the formula is PbI_2.

Solved When Lead Ii Nitrate And Potassium Iodide React Chegg Com

Equation For Pb No3 2 H2o Lead Ii Nitrate Water Youtube
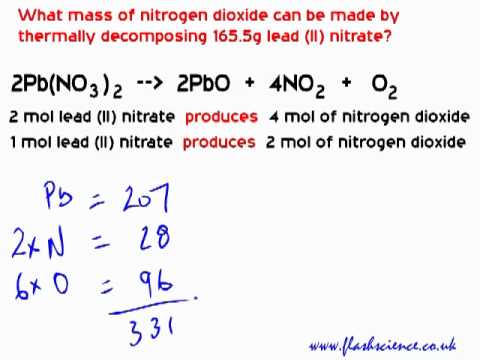
Reacting Mass Calculation Decomposition Of Lead Ii Nitrate Youtube

Lead Ii Nitrate Formula Updated For 2021 2022 Coolgyan Org
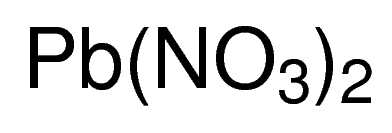
Lead Ii Nitrate 99 999 Trace Metals Basis 10099 74 8
Lead Ii Nitrate N2o6pb Chemspider

Acros Organics Ac211565000 Lead Ii Nitrate P A 500g From Masterflex

How To Write The Formula For Lead Ii Nitrate Youtube