Methylene chloride is predominantly used as a solvent in paint strippers and removers. Methylene chloride MC means an organic compound with chemical formula.
Analyze the extracts for the PAH of interest steps 10 through 18.

Vapor pressure of methylene chloride. Migration of volatile chemicals from the subsurface into overlying buildings is called vapor intrusion VI. Methylene chloride is slightly soluble in water and is nonflammable. Ammonium Chloride Ammonium Hydroxide.
Methylene Blue Nitric Acid. Methylene chloride dichloromethane is a solvent that can be found in paint removers. 440 mm Hg at 77 F NTP 1992 National Toxicology Program Institute of Environmental Health Sciences National Institutes of Health NTP.
Film Thickness mm Orientation Speed mmin Source Document Units Normalized Units. Industrially it is produced by the carbonylation of methylene chloride oxidation of vinylidene chloride or the addition of chlorine to ketene. Vapor Tightness of Gasoline Tank - Pressure Vacuum.
A 1001 dilution remedied any viscosity issues. Methylene blue is an organic chloride salt having 37-bisdimethylaminophenothiazin-5-ium as the counterion. Volatile organic chemicals in contaminated soils or groundwater can emit vapors which may migrate through subsurface soils and may enter the indoor air of overlying buildings.
Air to Fuel Burn Rates - Wood Fired Appliances. A commonly used dye that also exhibits antioxidant antimalarial antidepressant and cardioprotective properties. The values in the table below except as noted have been extracted from online and hardbound compilations.
Dichloromethane DCM or methylene chloride is an organochloride compound with the formula C H 2 Cl 2. National Toxicology Program Chemical Repository Database. Values for relative polarity eluant strength threshold limits and vapor pressure have been extracted from.
A table of properties for methyl chloride lists the saturation pressure as 1167 psia at 100 F. This colorless volatile liquid with a chloroform-like sweet odour is widely used as a solvent. Vapor or saturation pressure.
It has a role as an EC 1434 monoamine oxidase inhibitor an acid-base indicator a fluorochrome an antidepressant a cardioprotective agent an EC 3118. Oxygen Permeation at 35 C and 0 Relative Humidity through Polycaprolactone Film 12. Research Triangle Park North.
See reference for description of nonstandard test method. The collection sample should be whole blood in an arterial blood gas syringe with lyophilized heparin. For example methylene chloride paint stripper and perchloroethylene dry cleaning fluid are exempted compounds for outdoor regulation but they could pose serious health risks to exposed individuals if present indoors.
The bottom of the tube is no. NR Not. It may be prepared from chloroacetic acid and thionyl chloride phosphorus pentachloride or phosgene.
All electronic cigarette solutions were diluted with methylene chloride by one hundred fold. Polar molecules have a negative end and a positive end They tend to associate because the positive end of one molecule attracts the negative end of another molecule. Vapor Pressure Estimates - Using Antoine Equation One of the most common uses for vacuum pumps is to cause chemicals to evaporate.
2 The e. As a process solvent in the. Normalize the total mass of PAH found to the mass of sample collected.
Vapor Phase Organic Concentration in Waste Samples. Methylene chloride has an odor threshold of 250 parts per million ppm. With full facepiece operated in the pressure demand or other positive pressure mode.
The first is listed by the International Agency for Research on Cancer IARC as a potential human carcinogen and the second is listed as a probable human carcinogen. Properties of Organic Solvents. This dilution was carried out for the following reasons.
Accidental Release Measures Ventilate area of leak or spill. Hydrogen Halide and Halogen - Isokinetic Method. The physical properties.
Methylene chloride is metabolized in the liver to carbon monoxide which subsequently forms COHb. The routes of absorption can be dermal inhalation or oral ingestion. Methylene chloride is primarily used as a solvent in paint removers but is also used in aerosol formulations.
Vapor permeation rates do not usually contain a pressure differential. 19101052g3i Select and provide to. As fluid enters the head it makes the Dippy Bird top-heavy.
If a fluid consist of more than one component a solution components with. 1 Initial work with the e-cigarette solutions indicated the liquids were relatively viscous in nature. In contrast dichloromethane methylene chloride CH 2 Cl 2 is a polar molecule with a net polarity away from the partially positive carbon atom toward the partially negative chlorine atoms.
The vapor pressure for methylene chloride is 349 mmHg at 20 C and it has an octanolwater coefficient log K ow of 130. EPA proposed a ban on methylene chloride in paint and coating removal products during the final days of the Obama administration in early 2017. Christian Reichardt Solvents and Solvent Effects in Organic Chemistry Wiley-VCH Publishers 3rd ed 2003.
But how much vacuum is needed to make a certain chemical evaporate. Water diluted acid can react with metals to form hydrogen gas. Building depressurization may cause these vapors to enter the home through cracks in the foundation.
One way to estimate the vapor pressure of a given chemical compound is to use the Antoine Equation. The pressure exerted by the vapor phase is called the. Vapor or saturation pressure depends on temperature.
This viscosity resulted in the formation of air bubbles in the autosampler syringe. Hydrogen Halide and Halogen. At 100 F this table also lists h f g 15485 Btulbm and v f g 086332 f t 3 l b m.
Certification and Auditing - Wood Heaters. The table below provides. Water may be used to flush spills away from exposures and to dilute spills to non-flammable mixtures.
The temperature decrease in the head condenses the methylene chloride vapor decreasing the vapor pressure in the head relative to the vapor pressure in the abdomen. The Physical Property fields include properties such as vapor pressure and boiling point as well. Liquid travels to the head.
The vapor pressure of a liquid is defined as the pressure exerted by the molecules that escapes from the liquid to form a separate vapor phase above the liquid surface. Choose the solvent which gives the highest recovery of. Interim AEGLs for Methylene chloride 75-09-2 Exposure Period AEGL-1 AEGL-2 AEGL-3.
Employers who provide employees with gas masks with organic-vapor canisters for the purpose of emergency escape must replace the canisters after any emergency use and before the gas masks are returned to service. Example methylene chloride 34 and benzeneethanol 41 vv 5 have been recommended for extraction of PAH from diesel exhaust particulate. Although it is not miscible with water it is polar and miscible with many organic solvents.
Permeability Coefficient cm 3 μmm 2 day kPa Permeability Coefficient cm 3 mm. The greater vapor pressure in the abdomen forces fluid up through the neck and into the head.

Saturated Vapour Pressure Of Methylene Chloride As A Function Of Download Scientific Diagram

Saturated Vapour Pressure Of Methylene Chloride As A Function Of Download Scientific Diagram

Saturated Vapour Pressure Of Methylene Chloride As A Function Of Download Scientific Diagram

File Vapor Pressure Chart Svg Wikimedia Commons
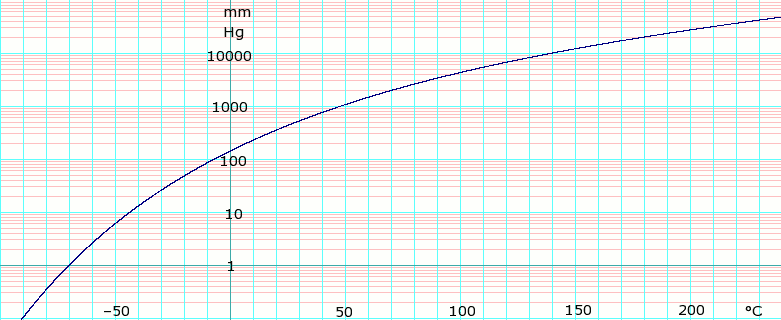
Dichloromethane Data Page Wikipedia

Chloromethane Data Page Wikipedia