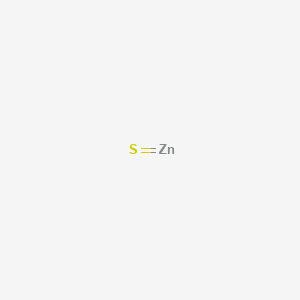Nitrate is NO 3- so nitrite has the same charge but one less oxygen NO 2- 2. Rongalite is a chemical compound with the molecular formula Na HOCH 2 SO 2.

How To Write The Formula For Zinc Sulfide Zns Youtube
Tin Il oxide 44silver sulfite.

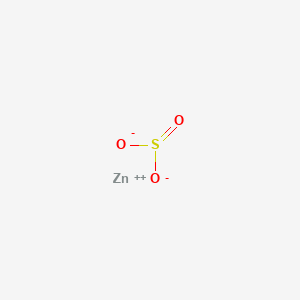
Zinc sulfite formula. Chemistry is all about learning chemical elements and compounds and how these things work together to form several chemical equations that are hard to understand. Potassium nitrate KNO3 11. Tin II bicarbonate SnHCO32 10.
Sodium thiosulfate ____Na2S2O3_____ 3. Write the chemical formula for the following ionic compounds. SnSO42 tin IV sulfate 55.
Zinc iron II iron III gallium silver lead IV chloride ZnCl 2. 2 Write the name for each of the following compounds. Sulfur dioxide ____SO2_____ 2.
Lead IV dichromate PbCr2O72 7. 2 lead IV sulfite 2. Cu2S copper I sulfide 43.
C 12 H 22 O 11. NH4NO2 ammonium nitrite 51. 2- so sulfite has the same charge but one less oxygen SO 3 2- b.
Strontium acetate SrC2H3O22 3. This salt has many additional names including Rongalit sodium hydroxymethylsulfinate sodium formaldehyde sulfoxylate and Bruggolite. Both anions show resonance in their chemical structures.
Sodium carbonate Na2CO3 6. The oxidation state of oxygen in. The first box is the intersection between the zinc cation and the chloride anion so you should write ZnCl 2 as shown.
Na Mg 2 Non. Both are anions bearing negative charges. The names are found by finding the intersection between the cations and anions.
Lead II chlorate PbClO32 2. Coating and surface treatment. 3 iron II sulfite 35 ZnNO 2 2 zinc nitrite 36 C 6H 12O 6 glucose 37 NiNO 3 2 nickel II nitrate 38 PCl 3 phosphorus trichloride 39 MnOH 7 manganese VII hydroxide 40 O 2 oxygen.
Fe 2 ferrous. One of the earliest methods of distinguishing between these ions used the suffixes -ous and -ic added to the Latin name of the element to represent the lower and higher oxidation states respectively. Aluminum hydroxide AlOH 3 1810 5 Aluminum phosphate AlPO 4 6310 19 Barium carbonate BaCO 3 5110 9 Barium chromate BaCrO 4 1210 10 Barium fluoride BaF 2 1010 6 Barium hydroxide BaOH 2 510 3 Barium sulfate BaSO 4 1110 10 Barium sulfite BaSO 3 810 7 Barium thiosulfate BaS 2 O 3.
MnClO44 manganese IV perchlorate 44. The overall charge of the anion is -2 for both anions. C 12 H 22 O 11.
Sr 2 strontium. Alganic acid or Na-Alginate. Solubility Product Constants near 25 C.
Radium sulfite souj IOK2Cr207 12PbBr2 Level Il 13ZnS03 14NaHC03 15. Students enrolled in Dr. H 2 SO 4.
The compound and its derivatives. If you know that a sufate ion is SO 4 2-then to get the formula for hydrogen sulfate ion you add a hydrogen ion to the front of the formula. Ionic Compound Formula Writing Worksheet Write chemical formulas for the compounds in each box.
Formula to name problems. Copper I sulfite Cu2SO3 8. Chemical Formula Writing Worksheet Two Write chemical formulas for the compounds in each box.
Zinc carbonate ZnCO 3 aluminum hypochlorite Aℓ C ℓO 3 calcium phosphate Ca 3PO 4 2 cadmium phosphate Cd 3PO 4 2 iron III sulfate Fe 2SO 4 3 mercury II chlorite HgC ℓO 2 2 potassium phosphite K 3PO 3 magnesium hydroxide MgOH 2 iron II chlorate FeC ℓO 3 2 cobalt II carbonate CoCO 3. The chemical formula of ionic compounds can be quickly calculated using the chemical formula calculator. ZnBr2 zinc bromide 45.
K2SO3 potassium sulfite 42. Chemical Formula 11 calcium bromate 12 carbon monoxide 13 potassium oxide 14 antimony tribromide 15 zinc phosphate. A 100-gram sample of a compound contains 365 grams of sodium 254 grams of sulfur and 381 grams of oxygen.
The first box is the intersection between the zinc cation and the chloride anion so you should write ZnCl 2 as shown. Al2S3 aluminum sulfide 54. Na 2 SO 3.
Section B Write the formula of the ionic compounds containing polyatomic ions 1. BaCIO32 barium chlorate 53. Draganjacs Introduction to Chemistry CHEM1003 General Chemistry I CHEM1013 and General Chemistry II CHEM1023 classes are responsible for learning the names and formulae for the common acids and common reagents and for learning the names formulae and the charges for the common cations and anions listed below.
Ionic Compound Formula K sp. The transfer of electrons between metals and non-metals produces charged particles called ions. Both anions are composed of a sulfur atom and oxygen atoms bonded to the sulfur atom.
7 Li2SO3 lithium sulfite 8 Zn3P2 zinc phosphide 9 SrC2H3O22 trontium acetates 10 Cu2O copper I oxide 11 Ag3PO4 silver phosphate 12 YClO3 yttrium chlorate 13 SnS2 tin IV sulfide 14 TiCN4 titanium IV cyanide 15 KMnO4 potassium permanganate 16 Pb3N2 lead. Chemical formulas can be. 1 NaF is sodium fluoride 2 K2CO 3 is potassium carbonate 3 MgCl 2 is magnesium chloride 4 BeOH 2 is beryllium hydroxide 5 SrS is strontium sulfide 6 Cu 2S is copper I sulfide 7 ZnI 2 is zinc iodide 8 Ca 3PO 42 is calcium phosphate 9 NH 4I is ammonium iodide 10 MnNO 33 is manganese III nitrate.
Ammonium cyanide NH4CN 5. H hydrogen. Chemical Formula Nomenclature Practice.
Fe 3 ferric. Copper Il acetate 42. Zinc phosphate Zn3PO42 4.
The names are found by finding the intersection between the cations and anions. And enables it to take a high finish. When the formula unit contains two or more of the same polyatomic ion that ion is written within parentheses and a subscript is written outside the parentheses to indicate the number of polyatomic ions.
Zinc Sulfite ZnSO 3 Magnesium Sulfite MgSO 3 Potassium Sulfite K 2 SO 3 Similarities Between Sulfate and Sulfite. Sodium chloride NaCl and magnesium oxide MgO. MgF2 magnesium fluoride 52.
1304-40-1 BaSi 2 O 5. An ionic compound is composed of a metal and a non-metal. Ca 2 calcium.
Sn 2 stannous. Complete these in lab and on your own time for practice. Does the empirical formula for the compound lead you to believe it is sodium sulfite o.
Li3P04 17SnC12 19Rb2Cr04 20KMn04 21CuCl 22FeS04 39chromium Ill chloride 40. A chemical formula shows the symbols of the elements in the compound and the ratio of the elements to one another. It is water-soluble and generally sold as the dihydrate.
Microsoft Word - Ionic_CovalentNameRace-1doc Created Date. Sulfate ion formula. It is listed in the European Cosmetics Directive as sodium oxymethylene sulfoxylate.
Metals lose electrons to produce positve ions called cations. You should complete this by Sunday. Give the formula for the following.
Some metals form positive ions in more than one oxidation state. Chemical formula plays an important role in understanding different concepts of chemistry. K potassium.
C 4 H 4 O 2. Parentheses and a subscript are not used unless more than one of a polyatomic ion is present in the formula unit eg calcium sulfate CaSO 4 not CaSO 4. Na-C 6 H 8 O 6n.
H 2 SO 3. Cations Anions zinc iron II iron III gallium silver lead IV chloride. Cadmium phosphate Cd3PO42 9.
Lithium sulfite 28 Zn 3 P 2 zinc phosphide 29 SrC 2 H 3 O 2 2 strontium acetate 30 Cu 2 O copper I oxide 31 Ag 3 PO 4 silver phosphate 32 YClO 3 yttrium I chlorate 33 SnS 2 tin IV sulfide 34 TiCN 4 titanium IV cyanide 35 KMnO 4 potassium permanganate 36 Pb 3 N 2 lead II nitride 37 CoCO 3 cobalt II carbonate 38 CdSO 3. Since a hydrogen ion has a 1 charge the net charge on. It gives paper a greasy or soapy feel.
Use the stock form for the transition metals. Students will write the formula and determine if compound is a strong or weak electrolyte.

Zinc Sulfide Formula Coolgyan Org

Compounds Vs Elements Compound Table Salt Soluble Crystals Stable Edible Elements Components Sodium Shiny Metal Reactive Poisonous Chlorine Ppt Download

How To Write The Formula For Zinc Sulfide Zns Youtube
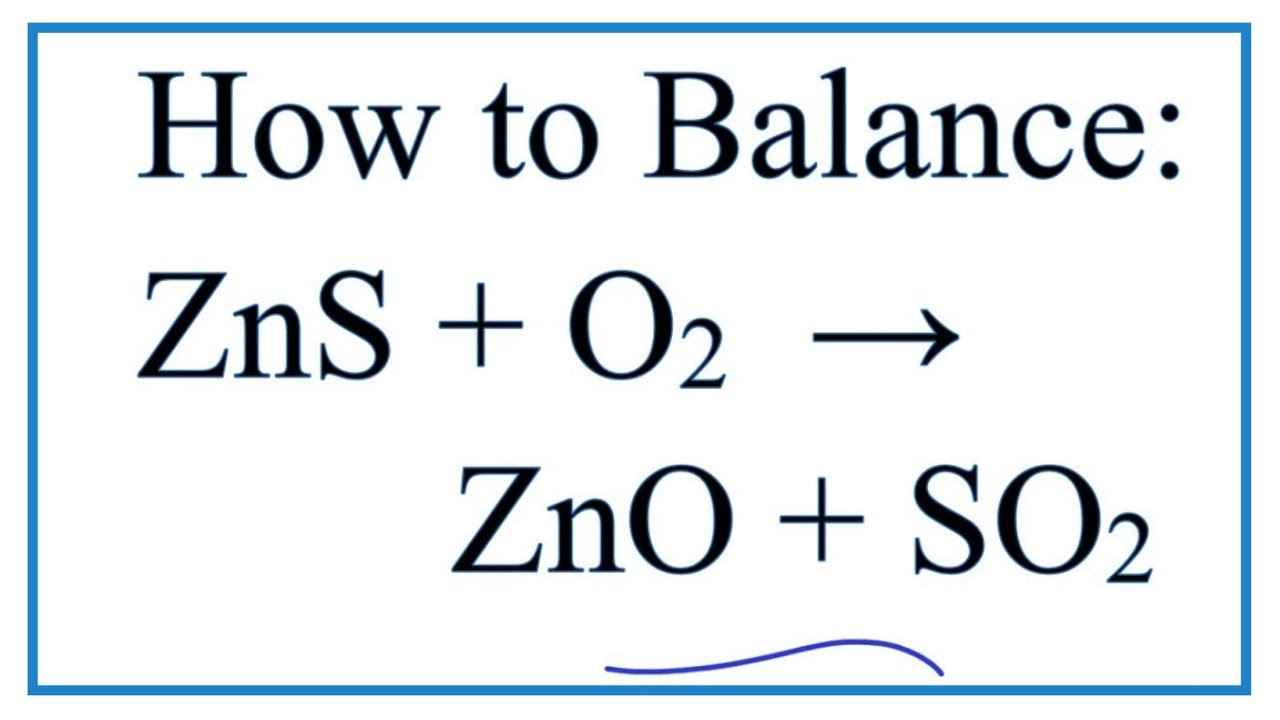
How To Balance Zns O2 Zno So2 Zinc Sulfide Oxygen Gas Youtube

How To Write The Formula For Zinc Sulfate Youtube