HAZARDS IDENTIFICATION Emergency Overview. When introduced to water sodium metabisulfite liberates.

How Much Sodium Sulfite Photo Net Photography Forums
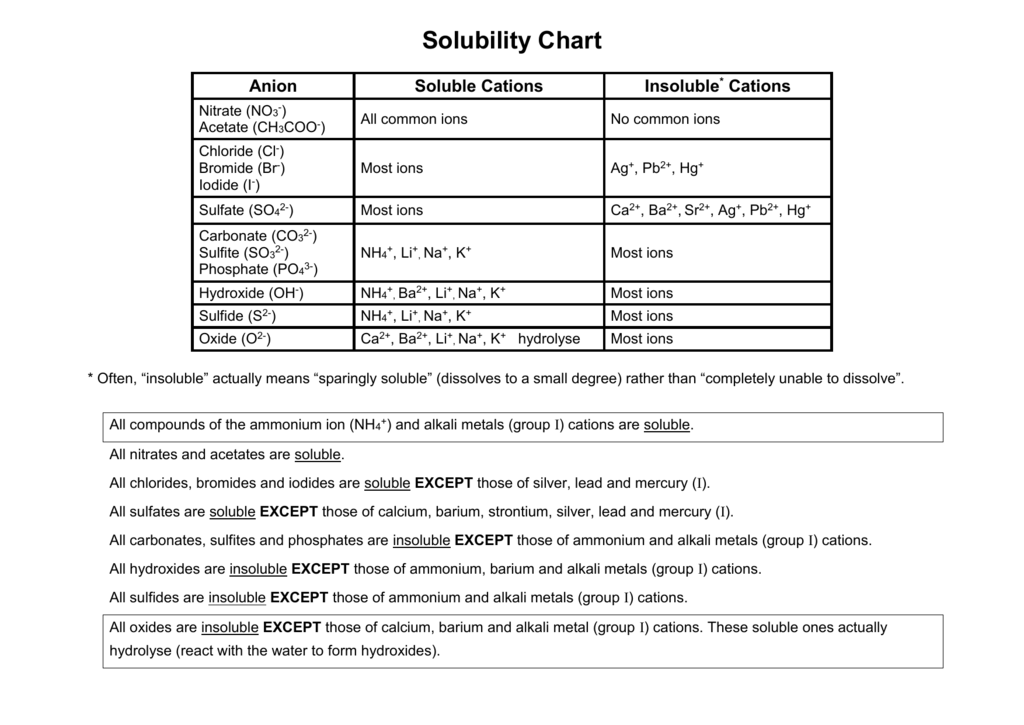
Most sulfites are insoluble in water.

Solubility of sulfite. Aging barrels should also be adequately maintained particularly when they are empty. M m A n s mM n aq nA m-aq. A slurry is a thick suspension of an insoluble precipitate in water.
Solubility product constant K sp or the solubility product is the product of the molar concentrations of the constituent ions each raised to the power of its stoichiometric coefficient in the equilibrium equationFor instance if a compound A a B b is in equilibrium with its solution. Solubility Product Constants near 25 C. This compound is highly soluble in glycerol but not very soluble in ethanol.
Trade Names and Synonyms. SOLUBILITY PRODUCT CONSTANTS The solubility product constant K sp is a useful parameter for calculating the aqueous solubility of sparingly soluble compounds under various conditions. A white water-soluble solid it is used commercially as an antioxidant and preservative.
Sodium sulfite sodium sulphite is the inorganic compound with the chemical formula Na 2 SO 3. The molar mass of sulfate is about 96 gmol. For ionic compounds with limited solubility in water an equilibrium constant K sp can be defined from the ion concentration in water from the equation.
Most sulfates are soluble in water. Acetic acid is a weak acid consequently it is written in molecular form. K sp M n m A m- n.
Significant attention must be paid to winery hygiene and equipment must be cleaned regularly during the harvest. Mainly anhydrous sodium sulfite is used for the removal of water from organic layer. GROUP I ALKALI METALS GROUP II ALKALINE EARTH METALS TRANSITION METALS POST-TRANSITION METALS Ammonium NH 4 Lithium Li Sodium Na Potassium K Magnesium.
A pale yellow aqueous solution with a pungent sulfur-like odor. Ammonium sulfite NH42SO3 479 54 608 688 784 104 144 150 153 Ammonium tartrate NH42C4H4O6 45 55 63 705 765 869 Ammonium thiocyanate NH4SCN 120 144 170 208 234 235 346 Ammonium thiosulfate NH42S2O3 173 205 269 Ammonium vanadate NH4VO3 048 084 132 178 242 305 70 Aniline C6H7N 36 Antimony trifluoride SbF3 385 444 562 dec Antimony sulfide Sb2S3. A saturated solution of sodium sulfite in water is mildly basic with an approximate pH value of 9.
From alkali metals only lithium forms insoluble carbonate. This website uses cookies to help provide you with the best possible online experience. The table below gives calculated values of K.
Sodium acid sulfite sodium hydrogen sulfite monosodium sulfite NaHSO3. FDA has ruled. CID 1100 Sulfurous acid CID 222 Ammonia Dates.
It is also a component in many drugs which helps maintain their potency and stability. Also called ammonia caramel. In aqueous solution it is only a few percent ionized.
It is corrosive to aluminum. Solubility is the property of a solid liquid or gaseous chemical substance called solute to dissolve in a solid liquid or gaseous solvent to form a solution of the solute in the solvent. Sulfite is an anion composed of sulfur and oxygen atoms.
Sodium sulfite is an inorganic salt with the chemical formula Na 2 SO 3. Sodium Sulfite is a white crystal or powder with reducing property. All salts of Group IA and ammonium are soluble.
Ag aq CH 3 COOHaq --- AgCH 3 COOs H aq Nitrate is the only spectator ion. Calcium sulfite or calcium sulphite is a chemical compound the calcium salt of sulfite with the formula CaSO 3 xH 2 O. All salts of nitrates chlorates and acetates are soluble.
Substance Substance name. Sodium sulfite exhibits bleaching de-sulfurizing and dechlorinating activities. 12 11062020 EN English US Page 1 SECTION 1.
Na 2 S 2 O 5 is fairly soluble in water its solubility corresponds to 653g100mL at a temperature of 20 o. So after separating the solvents residual water will remain the organic layer. With sulfite compounds sulfurous acid potassium sulfite potassium bisulfite sodium sulfite and sodium bisulfite.
All salts of carbonate phosphate and sulfite are. It may be determined by direct measure-ment or calculated from the standard Gibbs energies of formation f G of the species involved at their standard states. The solution itself is relatively non-toxic and.
Thus if K sp Mm An is the equilibrium. Silver acetate is insoluble and you learn this from a solubility chart. Most often the solvent is a liquid which can be a pure substance or a mixture.
NaClaq Sodium chloride Na-aq Cl aq K 24 SO aq Potassium sulfate 2 K-aq SO 4 2aq Li 23 CO aq Lithium carbonate 2 Li-aq CO 3 2aq Na 34 PO aq Sodium phosphate 3 Na-aq PO 4 3aq NH 42 SO 4 aq Ammonium. The molar mass of sulfite is about 80 gmol. Sodium Sulfite Anhydrous Safety Data Sheet according to Federal Register Vol.
Such a solution can undergo crystallization to yield heptahydrate crystals of Na 2 SO 3. Determinations of the solubility of a salt may be made by reference to SOLUBILITIES OF IONIC COMPOUNDS. In its anhydrous.
Non-combustible but excessive heat may liberate sulfur dioxide gas that is strongly irritating to the eyes and mucous membranes. Modern stainless steel equipment is easier to clean than tanks made of concrete or wood. It is a strong irritant to skin and mucous membranes.
Sodium Sulfite Anhydrous CAS-No. In addition the development of these microorganisms is favored by low doses of sulfite and high pH. Ionic Compound Formula K sp.
A heptahydrate is also known but it is less useful because of its greater susceptibility toward oxidation by air. No ammonium compounds are used. But when we study deeply about solubility of metal carbonates most of the carbonates are insoluble in water.
Ammonium bisulfite is colorless crystals which are soluble in water. Using the solubility guidelines provided in the lab manual for this experiment predict whether this stage of the scrubbing process will produce a slurry ie precipitate or a solution ie no precipitate of. One may also speak of solid solution but rarely of solution in a gas.
All forms are white solids. With ammonium reactant but no sulfite. It is an ionic compound containing two sodium cations Na and one sulfite anion SO 3 2-.
It is most notable as the product of flue-gas desulfurization. Water and dichloromethane is slightly soluble in each other. Anhydrous sodium sulfite is an insoluble.
Solubility of carbonates have a variation because there are soluble and insoluble carbonates. All salts of halides are soluble except those of silverI copperI leadII and mercuryI. It has a faintly pungent smell similar to SO 2.
Two crystalline forms are known the hemihydrate and the tetrahydrate respectively CaSO 3 ½H 2 O and CaSO 3 4H 2 O. All salts of sulfate are soluble except for barium sulfate leadII sulfate and strontium sulfate. Where M m A n is the slightly soluble substance and M n and A m-are the ions produced in solution by dissosiation of M m A n.
In the presence of ammonium compounds ammonium hydroxide ammonium carbonate ammonium hydrogen carbonate and ammonium. This agent was used by food industry to help maintain the fresh appearance of food products. Please read our Terms Conditions and Privacy Policy for information about.
Aluminum hydroxide AlOH 3 1810 5 Aluminum phosphate AlPO 4 6310 19 Barium carbonate BaCO 3 5110 9 Barium chromate BaCrO 4 1210 10 Barium fluoride BaF 2 1010 6 Barium hydroxide BaOH 2 510 3 Barium sulfate BaSO 4 1110 10 Barium sulfite BaSO 3 810 7 Barium thiosulfate BaS 2 O 3. Sodium sulfite has a white or whitish-yellow appearance in its solid-state. Soluble salts are written as their aqueous ions.
58 Monday March 26 2012 Rules and Regulations Issue date.

K2so4 Solubility And Sulfite Removal Operation Ranges In 5 Wt Aqueous Download Scientific Diagram

K2so4 Solubility And Sulfite Removal Operation Ranges In 5 Wt Aqueous Download Scientific Diagram
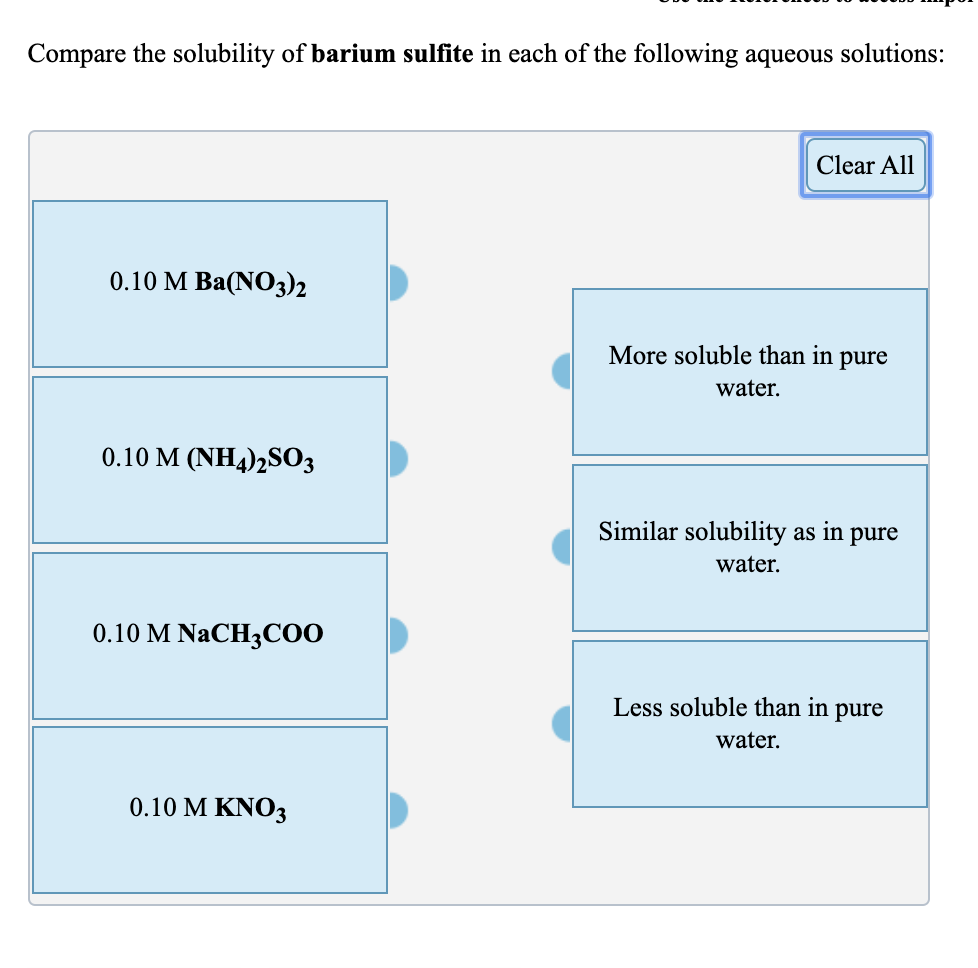
Solved Compare The Solubility Of Barium Sulfite In Each Of Chegg Com

Measurement And Correlation For The Solubility Of Sodium P Toluenesulfonate Sodium Sulfite And Sodium P Methylphenoxide In Aqueous Sodium Hydroxide Solutions And Sodium Sulfite In Aqueous Ethanol Solutions Journal Of Chemical Engineering Data
Sodium Sulfite Solubility Curve Pdf